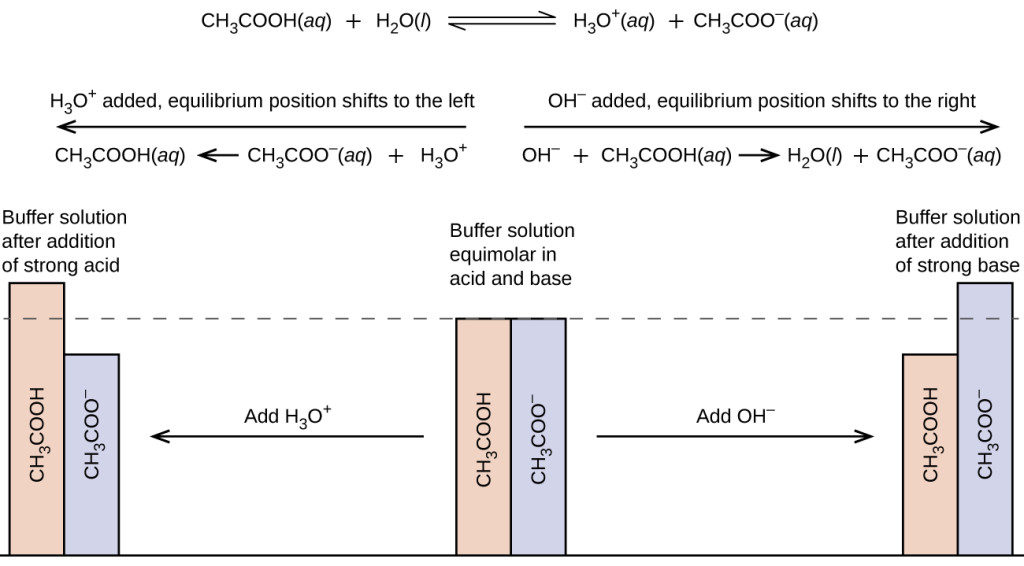
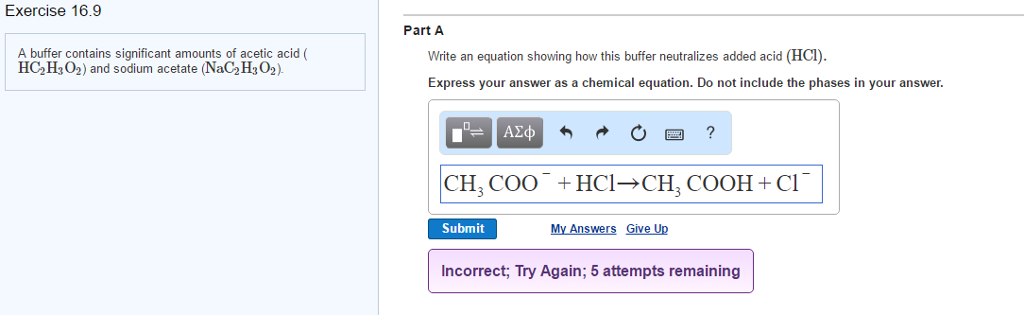
SOLVED: buffer solution is made from acetic acid, HCzH;Oz, and sodium acetate, NaCzH;Oz: Suppose small amount of hydrochloric What is the net ionic equation for the reaction that occurs when acid is

Preparation of acetate buffers using Sodium acetate and acetic acid using the Henderson-Hasselbach - Studocu

1 × 10^-3 mole of HCl is added to a buffer solution made up of 0.01M acetic acid and 0.10M sodium acetate. The final pH of the buffer will be: (given pKa
When a small amount of HCL is added to a buffer solution of acetic acid and sodium acetate what happen?

You have 250mL of a 0.56M solution of sodium acetate. How many mL of 0.50M acetic acid should be added to make a buffer of pH 4.40? | Homework.Study.com
Compare 1 L of acetate buffer solution (0.50 mol of acetic acid and 0.50 mol sodium acetate) to 1 L of HCl solution Similarities

Chapter 17 Acid-Base & Solubility Equilibria The Common Ion Effect 17.2 Buffer Solutions 17.3Acid-Base Titrations (omitted) 17.4Solubility Equilibria. - ppt download

E-Lifes: Acetate buffer preparation and calculation. (Weak acid + salt of weak acid , Weak acid + salt of weak acid, Weak acid + strong acid)