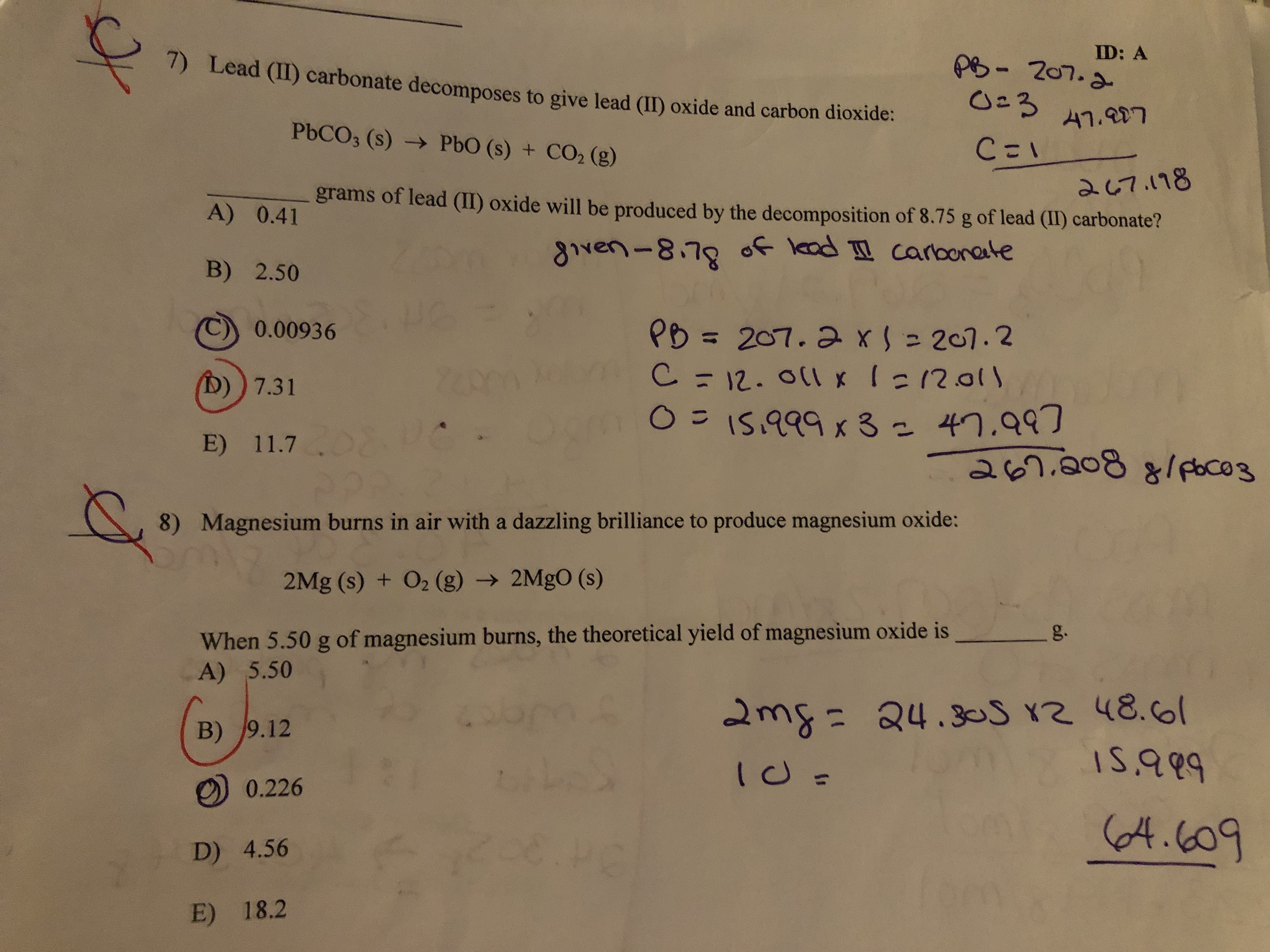A student was trying to extract the metals from lead oxide and aluminium oxide. She heated each oxide with carbon in a fume cupb
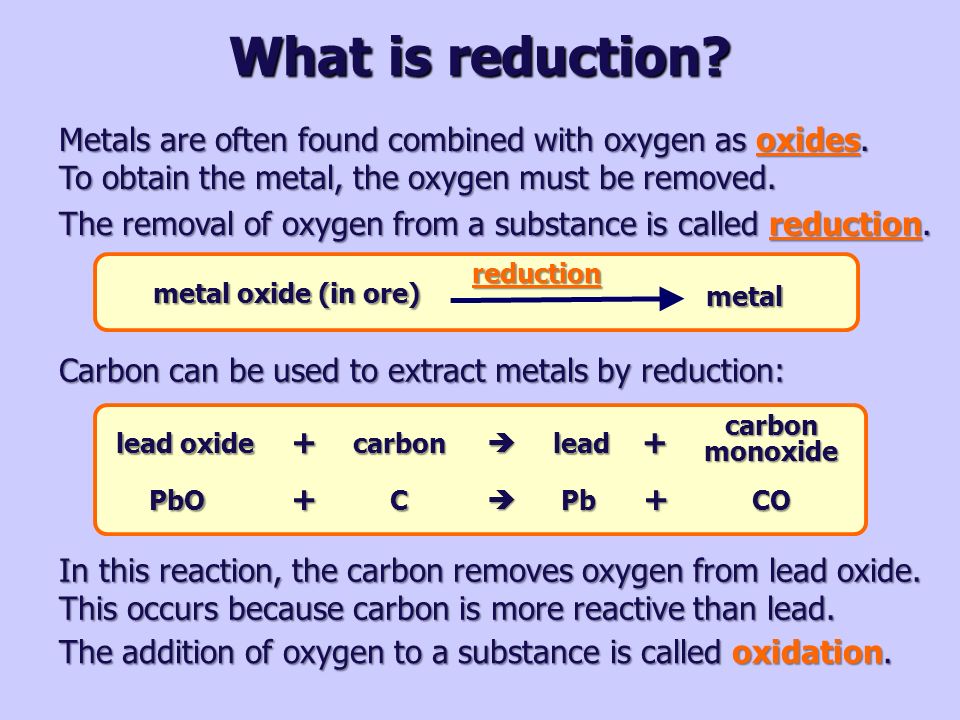
18 Which of the statements about the reaction below are incorrect? 2PbO(s)+C(s)→ 2Pb(s)+CO2(g) (a) Lead is getting reduced (b) Carbon Dioxide is getting oxidised (c) Carbon is getting oxidised (d) Lead oxide

What will be the product when lead oxide reacts with carbon i thought carbon - Science - Acids Bases and Salts - 12499069 | Meritnation.com
Dry carbon (II) oxide gas reacts with heated lead (II) oxide as shown in the equation below. Pb(s) - Tutorke

Which of the statements about the reaction below are incorrect ? 2PbO(s) + C(s) rarr 2Pb(s) + CO(2)(g) (a) Lead is getting reduced. (b) Carbon dioxide is getting oxidised. (c) Carbon is

SOLVED: Lead (II) carbonale decomposes t0 give lead (IIl) oxide and carbon dioxide: PbCO3 (s) + PbO (s) COz (g) grams of lead (II) oxide will be produced by the decomposition of

Which of the statements about the reaction below are incorrect ? 2PbO(s) + C(s) → 2Pb(s) + CO2(g) (a) Lead is getting reduced.(b) Carbon dioxide is getting oxidised.(c) Carbon is getting oxidised.(d)
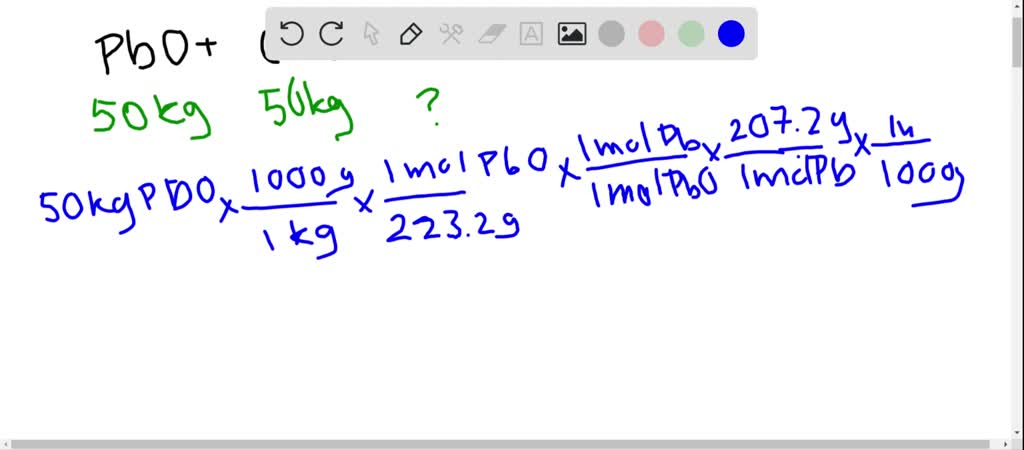
SOLVED:Lead(II) oxide from an ore can be reduced to elemental lead by heating in a furnace with carbon. PbO(s)+C(s) →Pb(l)+CO(g) Calculate the expected yield of lead if 50.0 kg of lead oxide

Preparation of High Purity Lead Oxide from Spent Lead Acid Batteries via Desulfurization and Recrystallization in Sodium Hydroxide | Industrial & Engineering Chemistry Research

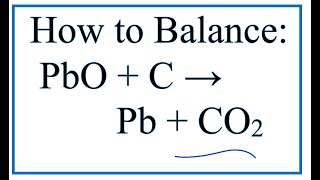
SOLVED: Lead can be produced by heating lead oxide with carbon. Complete the word equation for this reaction: *
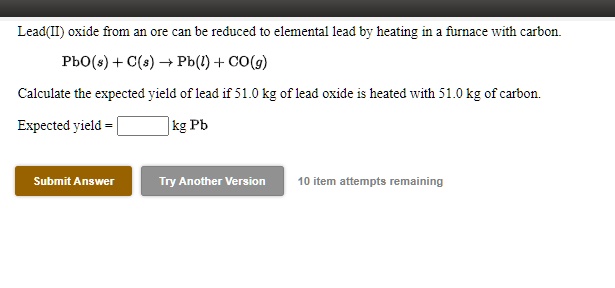
SOLVED: Lead(Il) oxide from an ore can be reduced to elementa) lead by heating in fnace With carbon Pbo(s) + C(s) = Pb(l) + CO(g) Calculate the expected yield of lead if
![For the given reaction,\\[2PbO + C \\to Pb + C{O_2}\\] name the oxidized substance, reduced substance, reducing agent, and oxidizing agent in this reaction. For the given reaction,\\[2PbO + C \\to Pb + C{O_2}\\] name the oxidized substance, reduced substance, reducing agent, and oxidizing agent in this reaction.](https://www.vedantu.com/question-sets/407a8186-d96f-4ae5-9b1b-a35a89f410b32918307568284777948.png)
For the given reaction,\\[2PbO + C \\to Pb + C{O_2}\\] name the oxidized substance, reduced substance, reducing agent, and oxidizing agent in this reaction.
Quiz (Extraction of Metals) 1. Metals are usually extracted from their ores before use. State the method of extraction for the f